CALUX® methods Endocrine disruption
Endocrine disruption has been recognized as a priority endpoint in safety evaluation of chemicals and consumer products. Endocrine disrupting compounds (EDCs) are being classified as substances of very high concern and regulations to restrict their use are being installed world-wide. EDC effects are hard to predict and are best tested using bioassays. To avoid animal studies BDS has developed a range of assays that allow screening and safety assessment of chemicals and complex mixtures including consumer products in a rapid and cost-effective manner.
Overview
CALUX® methods available for endocrine disruption screening
The unique CALUX® - based test panel includes
- Assessment of interferences with estrogen-, androgen- and thyroid pathways and steroidogenesis (EATS) using robust and quantitative human CALUX® reporter gene technology (1), and complementary assays
- Methods to measure in a wide variety of products and applications
- Used world-wide in major chemical-, pharmaceutical-, cosmetics-, food- and feed companies, and others
- Extensively validated to demonstrate robustness and predictivity
- Contract service or transferred to your laboratory via licensing and training
The high throughput screens are particularly suited for safe design, and in a regulatory context endocrine disruption. All methods can be combined with appropriate metabolic fractions (2).
Standard analyses performed according to OECD guidelines (for EA and S; under development for T), under ISO17025 and GMP+ certified conditions.
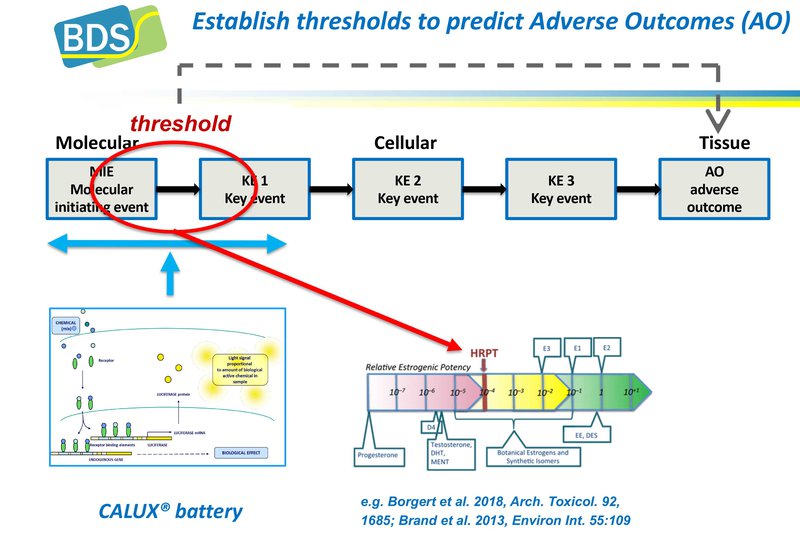
BDS’ assays are extensively validated, strictly quality controlled and link to a range of internationally accepted guidelines (OECD, ISO, EU). Being specific mechanism-based assays, the analytical results are straightforward and relatively easy to interpret. This is particularly the case for the endocrine assays, where linkage to reference methods is straightforward (3). In many cases this has allowed the definition of thresholds that can be used as points-of-departure in risk analysis.
Description of Assays
BDS develops and applies CALUX® mechanism-based high throughput screening panels for all major toxicity classes in REACH. In particular, the test battery for endocrine disruption is now able to comprehensively measure all endpoints of prime regulatory concern (as described in the ECHA/EFSA guidance document), i.e. androgen-, estrogen-, thyroid-, and steroidogenesis (EATS) effects, with- or without metabolic activation. All modules are linked to OECD guidelines or are subject to evaluation. The thyroid module includes receptor activation, ligand conversion and transport. Recently, the AR CALUX has been validated favourably by ECVAM and was included in OECD TG458. The battery of CALUX tests has been extensively used in a range of read-across case studies, including those aiming at regulatory application or in a safe design context (4-7). The CALUX methods have additionally been transformed from the classical manual format to high throughput format (384 wells) using robotics. Comprehensive panels of bioassays have been developed for safety- and activity profiling.
EATS Endocrine Disruption Panel
- E: (anti) Estrogens: ERalpha CALUX (OECD TG 455)
- A: (anti) Androgens: AR CALUX (ECVAM validation, OECD TG 458)
- T: Thyroid interference: TRbeta CALUX, TTR and TOP assay (TG in preparation)
- S: H295R steroidogenesis (OECD 456)
- Phase 1 and Phase 2 metabolic steps
Approach
Based on its CALUX reporter gene technology and focusing on the EATS mode of action of EDCs BDS has developed a range of specific assays that allow screening and safety assessment of chemicals and consumer products in a rapid and cost-effective manner. Complementary assays have been selected to complete the comprehensive panel.
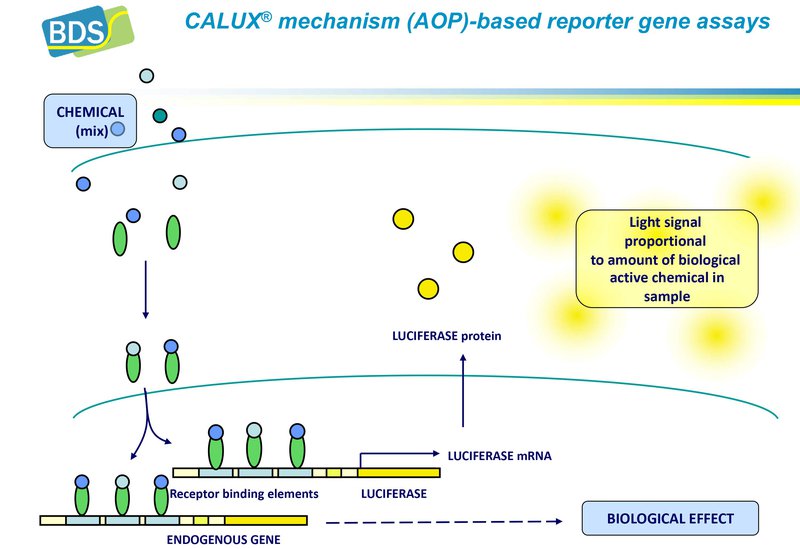
These assays have been extensively validated and most of them already incorporated in relevant OECD test guidelines. These OECD guidelines define thresholds based on the threshold between negative and positive compounds. In addition thresholds have been defined for specific applications such as water quality monitoring, thereby assessing the effects of complex mixtures. In addition, methods have been used for safe design of new chemical entities. Methods can be used with and without modular metabolic systems.
In Silico Evaluation and Reporting
An initial compound evaluation based on existing data and models is available as a report service. The analysis follows EcHA guidance on the analysis of existing data and models and recommendations for in vitro testing following OECD guidance. When desired by the customer, detailed harmonized datasets and analysis can be provided with testing result reporting.
Regulatory Guidance
A regulatory guidance service is provided supporting the preparation of reporting information for regulatory purposes (e.g., REACH reporting).
VISIT OUR SHOP
- Van der Burg, B., Van der Linden, S.C., Man, H.Y., Winter, R., Jonker, L., Van Vugt-Lussenburg, B., Brouwer, A. (2013) A panel of quantitative CALUX® reporter gene assays for reliable high throughput toxicity screening of chemicals and complex mixtures. In "High throughput screening methods in toxicity testing" (P. Steinberg, ed). John Wiley and Sons, Inc. New York. ISBN 9781118065631
- van Vugt-Lussenburg, B.M.A., van der Lee, R.B., Man, H.Y., Middelhof, I., Brouwer, A., Besselink, H., van der Burg, B. (2018) Incorporation of metabolic enzymes to improve predictivity of reporter gene assay results for estrogenic and anti-androgenic activity. Reprod. Toxicol. 75:40-48.
- Van der Burg, B., Van der Linden, S.C., Man, H.Y., Winter, R., Jonker, L., Van Vugt-Lussenburg, B., Brouwer, A. (2013) A panel of quantitative CALUX® reporter gene assays for reliable high throughput toxicity screening of chemicals and complex mixtures. In "High throughput screening methods in toxicity testing" (P. Steinberg, ed). John Wiley and Sons, Inc. New York. ISBN 9781118065631
- Van der Burg, B., Van der Linden, S.C., Man, H.Y., Winter, R., Jonker, L., Van Vugt-Lussenburg, B., Brouwer, A. (2013) A panel of quantitative CALUX® reporter gene assays for reliable high throughput toxicity screening of chemicals and complex mixtures. In "High throughput screening methods in toxicity testing" (P. Steinberg, ed). John Wiley and Sons, Inc. New York. ISBN 9781118065631
- van Vugt-Lussenburg, B.M.A., van der Lee, R.B., Man, H.Y., Middelhof, I., Brouwer, A., Besselink, H., van der Burg, B. (2018) Incorporation of metabolic enzymes to improve predictivity of reporter gene assay results for estrogenic and anti-androgenic activity. Reprod. Toxicol. 75:40-48.
- Sonneveld, E., Riteco, J.A.C., Jansen, H.J., Pieterse, B., Brouwer, A., Schoonen, W.G., Van der Burg, B. (2006) Comparison of in vitro and in vivo screening models for androgenic and estrogenic activities. Toxicol. Sci., 89,173-87.
- Van Vugt-Lussenburg B, Van Es D, Naderman M, le Notre J, van der Klis F, Brouwer A, Van der Burg B. (2020) Endocrine activities of phthalate alternatives; assessing the safety profile of furan dicarboxylic acid esters using a panel of human cell based reporter gene assays. Green Chemistry 22, 1873-1883