KeratinoSens™ by Curio Biotech Ltd
Curio Biotech Ltd
Founded in Jan 2017, Curio Biotech Ltd is a GLP ready Swiss Contract Research Organization (CRO) located at BioArk (biotech park), Visp, Switzerland. It provides in vitro testing services to Cosmetic, Pharma (small and large molecules) and Diagnostic industries using proprietary culture media and primary human cell based 3D co-culture models. In vitro services focus on basic research, drug discovery, toxicity, pharmacology in the areas of Dermatology, Immuno-oncology and Regenerative Medicine.
Furthermore, Curio Biotech offers a variety of batch/lot release tests like potency tests for Bio-pharma industry, Biocompatibility tests and other regulatory OECD tests.
Allergic contact dermatitis (ACD) is a common skin disease with a significant socio-economic impact. ACD is induced by repeated skin contact with an allergen (hapten, pro-haptenor complete allergen). The assessment of the skin sensitizing potential of haptens such as industrial chemicals, agrochemicals, and cosmetic ingredients is crucial in order to define their safe handling and use. With the ban on use of animals for cosmetics testing in Europe, USA and several other countries, the application of in vitro methods have become indispensable for the hazard assessment of chemicals used in consumer skin care products.
An Adverse Outcome Pathway (AOP) is an analytical construct that describes a sequential chain of causally linked events at different levels of biological organisation that lead to an adverse health impact. AOP for skin sensitisation initiated by covalent binding of chemicals to proteins, assesses the weight-of-evidence supporting the AOP, identifies the key events (KE), and identifies databases containing test results related to those key events. AOPs can be incorporated into chemical category-based assessments or integrated approaches for testing and assessment.
The following are the KE of AOP leading to skin sensitisation after skin penetration (OECD Guideline test No.428. OEC).
| KE 1 | Haptenation-attachment of allergen to skin protein | OECD Guideline No. 442C |
|---|---|---|
| KE 2 | Epidermal inflammation-release of pro-inflammatory signals by epidermal keratinocytes | OECD Guideline No. 442D |
| KE 3 | Immune recognition of allergens, Dendritic cell (DC) activation and maturation | OECD Guideline No. 442E |
| KE 4 | T-cell proliferation: clonal expansion of hapten-peptide specific T-cells |
KeratinoSens™ grown in fetal bovine serum containing medium
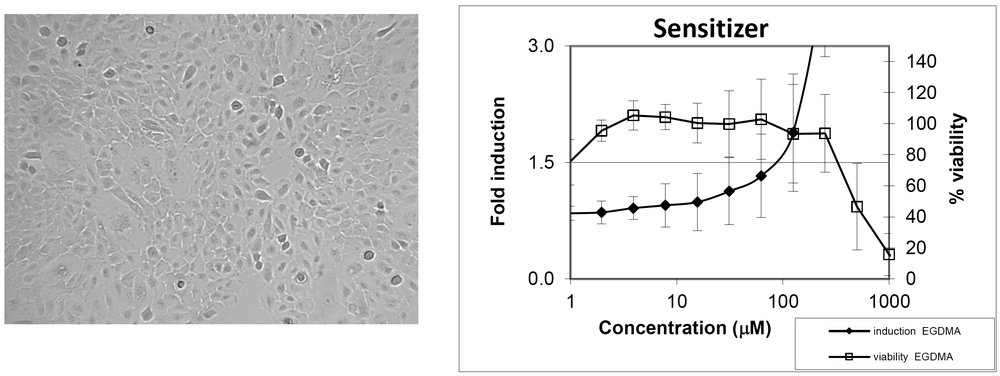
The KeratinoSens™ cell line addresses KE 2 that occurs in the epidermal keratinocyte gene expression associated with specific cell signalling pathways. The KeratinoSens™ cell line is a cell line derived from human keratinocytes that contains a stably transfected luciferase gene under the transcriptional control of a constitutive promoter fused with an ARE (an antioxidant/electrophile response element). Once a sensitizer binds to the ARE, the luciferase gene is upregulated.
Upregulated luciferase activity is measured using a standard substrate. Based on the results, the test chemical is classified as a Positive sensitizer if the activity is more than or equivalent to 1.5 fold induction or as Non-Sensitizer if it is less than 1.5 fold induction.
| Cell Line | KeratinoSens™ cell line |
|---|---|
| Assay Protocol | The KeratinoSens™ cell line is seeded and grown for 1 day either in Fetal Bovine Serum or Xeno-free (human serum) based cell culture medium. Treatments with the test compounds for 48 hours, then cell viability is evaluated using Resazurin and luciferase reporter gene expression through ONE-Glo Luciferase Assay System. |
| Required Test Samples | 10ml of liquids / 10g of solids |
| Application and Test samples |
Three independently performed experiments 12 concentrations in triplicate 2000 μM as highest concentration (as per OECD guideline) |
| Quality Controls |
Vehicle control: 1% DMSO, medium Reference control: cinnamic aldehyde |
| Time Point | 48 hours |
| Data Analysis | Cell viability and induction of luciferase reporter gene expression |
| Data Delivery |
Imax Maximal fold induction (maximum response) EC1.5 value of luciferase activity IC50 values of cell viability Dose response curves for luciferase induction and cell viability |
| Sensitizer prediction |
Significant induction of luciferase activity >1.5 fold EC1.5 is <1000μM Cell viability >70% Dose response for luciferase induction |
- OECD Guidelines for Testing of Chemicals, number 442d “In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method” (adopted: June 25, 2018).
- United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS), Sixth revised edition, UN New York and Geneva. UN (2015),
- Emter R., Ellis G., Natsch A.(2010). Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicology and Applied Pharmacology 245, 281-290