Silensomes™
Silensomes™ are irreversibly inactivated pooled human liver microsomes (HLM) in which cytochromes P450 (CYP) enzymes have been chemically knocked down using mechanism-based inhibitors (MBI). Silensomes™ is available as a ready-to-use product, where CYP 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4 can be individually chemically knocked-down. The technology guarantees over 80% inhibition of the targeted CYP and less than 20% off-target impact on the other CYPs.
Drug-drug interaction can significantly impact drug safety and efficacy. Prediction of the risk of drug-drug interaction is part of the development of a new drug candidate and the registration dossier. In vitro identification and measurement of the contribution of the major CYPs involved in the human metabolism of a new drug candidate, also called “CYP phenotyping”, helps predicting the impact of a co-administered drug (perpetrator) on the pharmacokinetics of a new chemical entity (victim).
Currently, for CYP phenotyping, a battery of in vitro tests (antibody or chemical inhibition, estimation of fraction metabolized by recombinant human enzymes, and correlation analysis) recommended by the regulatory agencies (EMA/FDA) is required. The major drawbacks of the battery are lack of quantitative measurement of the contribution of each CYP in the metabolism of a drug (correlation analysis), lack of specific commercially available anti-CYP antibodies and last but not least human recombinant CYPs do not fully represent the in vivo liver enzymes.
To overcome the listed limitations, the Silensomes™ product was developed (patent owner Servier Laboratories, world-wide licensee Biopredic).
Silensomes™ is the model of choice for determination of the CYP contribution all along the development plan of a new chemical entity. It is a unique solution for a direct quantitation of the fraction metabolized (fm (f for fraction, m for metabolized)) by different hepatic CYP enzymes. Main advantages of the product are:
- Irreversible inhibition of CYPs, using mechanism-based inhibitors
- Possibility for parallel incubation with control HLMs
- Quantitative data: the true contribution of the 9 main CYP450s in the metabolism of the compound
- Better representation of in vivo situation by using human liver microsomes in comparison to the use of recombinant enzymes
- One single model and protocol for CYP phenotyping assays, instead of a battery of in vitro tests. The 9 main CYPs involved in the drug metabolism are included in the Silensomes™ line of products
Silensomes™ are relevant for both compound screening and for regulatory filing.
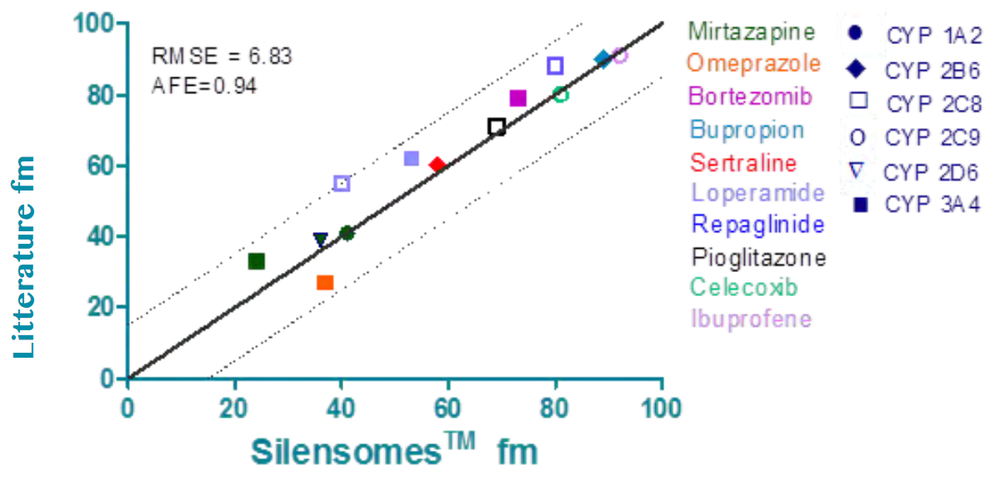
Good correlation between the in vivo Fm and the calculated in vitro Fm using Silensomes™. The analysis was done on 10 multi-CYP substrates and showed that for all tested compounds, the in vitro CYP contributions (fm) obtained with Silensomes™ correlate very well with the in vivo values.
A simple and at the same time content-rich assay
- In one assay you can:
- Obtain the Intrinsic clearance of your compound
- Determine which CYPs are involved in the biotransformation
- Quantify the CYPs involved.
VISIT OUR SHOP
Order Silensomes™from Biopredic International
Parmentier et al. (2018) Xenobiotica, doi: 10.1080/00498254.2017.1422156