New Approach Methods in Safety Assessment EU-ToxRisk Solutions
Who we are
The EU-ToxRisk Commercial Partnership is a joint venture between organisations providing coordinated integrated solutions to industrial problems in safety assessment. The partners bring experience and resources together to provide a one-stop shop in safety assessment solutions.
We jointly have experience in New Approach Methodology in safety assessment, predictive toxicology, in vitro screening, computational modelling, toxicogenomics, read across, tiered strategies, data science, product design, risk assessment and regulatory assessment.
Our Expert Team
- Barry Hardy, Edelweiss Connect GmbH, coordinator
- Costanza Rovida, CAAT-Europe
- Bart van der Burg, BDS
- Bas ter Braak, Leiden University
- Monika Kijanska, InSphero
- Paul Jennings, Vrije Universiteit Amsterdam
- Gerhard Ecker, Phenaris
- Andras Dinnyes, Biotalentum
What we provide
Solutions to:
- Tiered strategies in safety assessment
- Evaluation of toxicological endpoints: e.g., liver, kidney, neurotoxicity, skin, eye, endocrine disruption
- Integrated screening and data analysis across multiple technologies
- High quality best practices in protocols, data generation and processing
- Data mining and modelling to supporting decision making and jobs to be done
- Product ingredient screening and evaluation
- Formulation testing and assessment
- Animal free safety assessment
- Read Across
- Risk Assessment
- Regulatory reporting
Adverse Outcome Pathways (AOPs)
- Knowledge-mining of background information and documents to propose AOP key events, related hypotheses and testing strategies
- In vitro and toxicogenomics testing and analysis of AOP-based hypotheses
Testing
- Our in silico modelling and in vitro testing workflows are anchored to key events of Adverse Outcome Pathways (AOPs)
- Our testing is guided by in silico modelling e.g. we can provide a business case for testing based on the value of new information generated
- We can carry out exhaustive searches of existing databases, public knowledge sources, and document repositories using data and knowledge mining techniques supported by expert curation capabilities as required
Modelling
- We can apply a variety of algorithms and machine learning techniques to datasets supporting the building of reference and customised models
- For skin sensitization we have reproduced the leading defined approaches developed and used at industry leaders leaders such as P&G, BASF, Givaudan and Shiseido and including a well-documented and easy-to-use comparison of model results
- We also can apply our computational chemistry expertise to the evaluation of properties such as metabolism and transporters
Contact us with your problem and we will work together to identify your needs and coordinate a response with a solution!
Our Assets
Data Management
- Our EdelweissData™ approach to data management organises all data for a project or customer order using best data practices and standards
- All metadata and data is harmonised and aims for completeness and integrity
- Data processing workflows are organised as reproducible in silico protocols with instances stored within the system for future reference
- All in silico and in vitro protocols are fully described and stored in the system
- Secure Customer Data management solutions can be provided through premium customer accounts
Consulting
- We offer leading consulting expertise to all customer projects including case study design, in silico modelling, testing and report preparation
- Our in silico modelling and in vitro testing are planned and executed by our case study leader in a tiered manner
- We have significant experience in coordinating safety research or infrastructure development projects, building data management and software solutions, applying machine learning and AI to larger datasets and knowledge collections
- We have leading experience in Alternatives to Animal Testing, 3Rs, New Approach Methodologies, and related regulatory guidance issues and can help solve difficult issues that customers have in addressing information requirements of regulations (e.g. REACH)
Assays
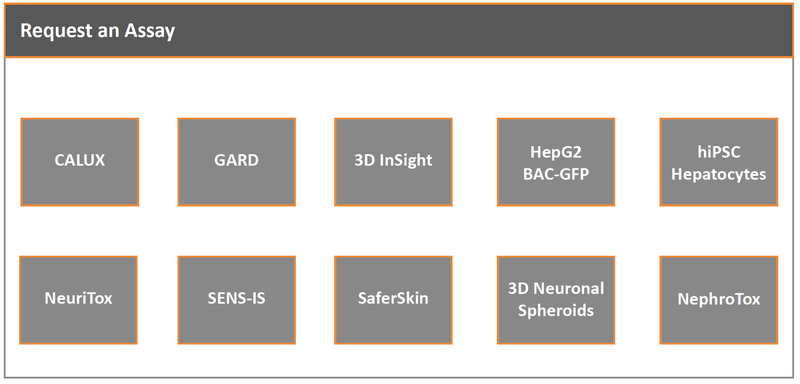
Specialist Areas
- Characterisation and risk assessment of nanomaterials including addressing emerging regulatory information requirements
- Systemic Toxicity evaluation of cosmetic ingredients e.g., including mechanistic liver and kidney assessment based on New Approach Methodologies
- High throughput safety evaluation of chemicals and chemical mixtures
- Transporter and metabolism modelling
- 3D liver cell culture screening and high content imaging
AI and Machine Learning
- We have leading expertise in developing and validating QSAR models
- We apply our machine learning expertise to the development of models in applications including customised versions for customers
- Our AI team has leading expertise in extracting targeted knowledge from information collections (documents, abstracts etc.) and organising it as structured information e.g., available to applications from a customised EdelweissData™ instance
References
- Our reference community version of SaferSkin™ demonstrates reproduction of best practices and methods at leading industrial organisations such as Procter and Gamble, BASF and Givaudan
- We have developed the knowledge infrastructure for leading international programs supporting the development of new methods in safety assessment such as EU-ToxRisk
- High throughput safety evaluation of chemicals and chemical mixtures
- We developed the knowledge infrastructure for leading European programs supporting the development of new methods in the safety assessment of nanotechnology such as eNanoMapper and ACEnano
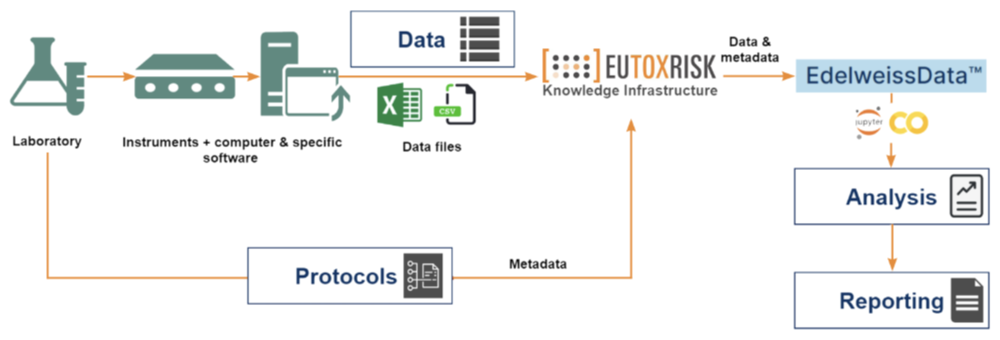
Partner Offerings
EdelweissConnect
- Organisation of main contact point with customers for coordinating responses of the Parties
- Coordination of EU-ToxRisk commercial response desk responding to customer enquiries
- Coordination of legal and business processes and documentation for commercial proposal preparation and service provision
- Coordination and project management of commercial projects
- EwC’s EdelweissData™ infrastructure supporting project data management and analysis
- EwC safety assessment laboratory in Basel
- EwC science and technical teams for application development, consulting and safety assessment support
- EwC marketing and sales functions operating out of Basel for Europe and Raleigh-Durham for US
See more on EdelweissData
Vrije Universiteit Amsterdam

- Testing of substances in renal epithelial cells (cell line, iPSC or primary) and toxicity assessment
- Mitochondrial toxicity assessment
- Phase I and Phase II Metabolism in human liver extracts and/or recombinant preparations
- Molecular docking, molecular dynamics and site of metabolism prediction
- Consultation on most appropriate in vitro models and assays
See more on Mechanistic toxicology
BioDetection Systems

- Laboratory services, including proprietary CALUX® methods measuring the interaction of compounds with key toxicological targets in human cells
- Quantitative high throughput analysis for safety assessment of chemicals and a wide variety of consumer products in a rapid and cost-effective manner
- Extensively validated and incorporated in relevant international (OECD,ISO,EU) guidelines
- Marketing and scientific information and support to the Coordinator
- Support for customer relationship establishment, negotiation, business planning and project or service delivery
See Endocrine disruption: Calux® methods
Phenaris
- Computational models for predicting interaction of compounds with transporters related to ADMET
- Data, models, and decision support in all aspects of in silico toxicology
- Decision support based on toxicological read across
See more on Transporter Modelling
InSphero

- 7 or 14 day cytotoxicity assay based on 3DInSight™ Human Liver MicroTissues
- Causality assays covering reactive metabolite formation, oxidative stress, and inflammation
- Consulting (in the area of investigative toxicology based on 3DInSight™ Human Liver MicroTissues)
See more on 3D InSight™ Human Liver Microtissues
CAAT-Europe
- Organization of communication and dissemination activities, as workshops and information days targeting different groups (regulators, industry, academia)
- Risk assessment in the scope of REACH or other EU Regulations
- Testing in unique neurotoxicity and DNT tests, as individual tests or in combination with other provided test methods, namely:
- NeuriTox, PeriTox, cMINC
- Full characterization of different cell systems (neuro, kidney, liver) for the following key events: cell death, energy metabolism, oxidative stress.
See more on how to Understand the toxicological profile
See more on Developmental Neurotoxicity (DNT)
University of Leiden

- HepG2 BAC-GFP reporter technology with panel of >50 human reporter cell lines
- High content screening for dynamic stress pathway activation
- Automated high content image analysis with single cell resolution
- Compound prioritization using reporter cell lines
- Culturing of a range of cell types including pooled donor primary human hepatocytes (PHH), HepG2, hiPSC, hiPSC-derived hepatocytes
- Exposures of cell cultures to chemicals, cell harvesting, sequencing and transcriptomic analysis
- Toxicological pathway using reporter cell lines
BioTalentum

- Toxicity assay (acute or 7 days long chronic exposure) based on 21 and 42 days old hiPSC derived neuronal cultures
- Toxicity assay based on 3D neuronal spheroids at various differentiation stages (acute exposure) representing fetal development
- Consulting (in the area of investigative neurotoxicity and DNT based on human neuronal models) and trainings on the methods above
See more on Human Induced Pluripotent Stem Cells
Contact us with your problem and we will work together to identify your needs and coordinate a response with a solution!
Eu-ToxRisk is an Integrated European ‘Flagship’ Programme Driving Mechanism-based Toxicity Testing and Risk Assessment for the 21st century.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant agreement N° 681002